Elements within the same group in the periodic table have similar properties because they have the same number of A. Block Elements are organised into blocks by the orbital type in which the outer electrons are found.

Physical Properties Of Elements
The number equal to the number of protons in an atom that determines its chemical properties.

. It forms covalent bonds because it wants four more electrons to share in order to fill its outer shell. The correct option is 3. Good conductors of heat.
They are arranged in rows which lists their atomic number. Which is not a family of the. Elements which appear in the same column have similar properties periodicity.
For example all of the elements in group XVII 17 the Halogens all react in a similar fashion. The periodic table shows the elements C in the same columns that typically have similar properties. In the Periodic Table the elements typically are in the same column have similar properties same valence electrons and the same charge.
Group The elements in group 1 of the periodic table are the _________ They include lithium sodium potassium rubidium cesium and francium. Elements in the same group of the periodic table have similar properties because their electronic configurations have the same number of electrons in the outermost shell. Metals tend to be A.
Which contains elements with similar properties in. Image of the standard periodic table of the elements Standard form of the periodic table. Period A horizontal row in the periodic table.
Elements in the same group usually have similar properties because they have the same number of electrons in the outermost electron shell. Does carbon typically form covalent or ionic bonds and why. For example Sodium potassium lithium rubidium and caesium have similar chemical properties.
All elements within a certain group thus share similar properties. Carbon is in Group 4 on the periodic table. These columns are called groups.
Elements in the same column of the periodic table in the Groups labeled A tend to have similar chemical and physical properties because they have the same number of A. Thus the right option is E. Moreover elements in group two of the periodic table are called the alkaline.
The elements is a group on the Periodic Table are considered a family because they have similar electron configurations and similar properties. Similar chemical properties only Explanation. While there are radioisotopes of other elements all of the actinides are radioactive.
People also ask why do elements in the same have. Elemental behavior is almost completely reliant on this outermost shell configuration with inner shells playing a less important role in determining properties. Elements in the same group of the periodic table are known to possess similar chemical properties.
Elements with similar chemical and physical properties are found in the same group of the periodic table. Chemistry questions and answers. There are eight main groups of elements numbered 1 2 and 13-18.
The elements in group 2 the second column form compounds consisting of one atom of the element and two atoms of hydrogen. A vertical column in the periodic table which signifies the number of valence shell electrons in an elements atom. Elements with three electrons or.
They all like to attract one additional electron and form a -1 anion. For instance Calcium and Magnesium have 2 electrons in their outermost shell compared to other elements listed. In their pure state all of these elements tend to have a shiny metallic appearance.
Elements in the same group of the periodic table typically have O similar atomic masses similar physical and chemical properties O similar chemical properties only O similar physical properties only Osimilar mass numbers. Very hard usually shiny ductile and malleable. Platinum group - Wikipedia A group is any column on the periodic table.
In the periodic table which. These columns are called groups. These elements except hydrogen are known as alkali metals and they all have similar chemical properties.
The atomic number of each element increases by one reading from left to right. Also the number of the electron in their outermost shell are equal. Elements that have similar chemical properties are grouped in columns called groups or families.
Members of a group typically have similar properties and electron configurations in their outer shell. These are called alkaline earth metals with similar properties. View the full answer.
Elements that share properties of both metals and nonmetals are called A. The periodic trend states that elements in the same group column in the periodic table have similar properties. Therefore Elements with similar chemical properties have D the same number of electrons in an outermost shell as the elements in the same group only have the same number of electrons in the outermost shell.
The tendency of an atom to attract electrons in a bond. Note that the standard form of the periodic table usually has hydrogen H located in Group 1 despite it being a gas while the other compounds are metals. Metals in the same _____ in the periodic table usually have very similar chemical properties.
The chemical properties of elements in the vertical groups are similar in some respects to each other. Nitrogen is in Group 15 and has five valence electrons. In the periodic table which elements typically have similar properties.
As well as being numbered some of these groups have namesfor example alkali metals the first column of elements alkaline earth metals the second column of elements halogens the next-to-last column of elements and noble gases the last column of. O those on opposite sides O those in the same columns O those in the same rows O those related diagonally.
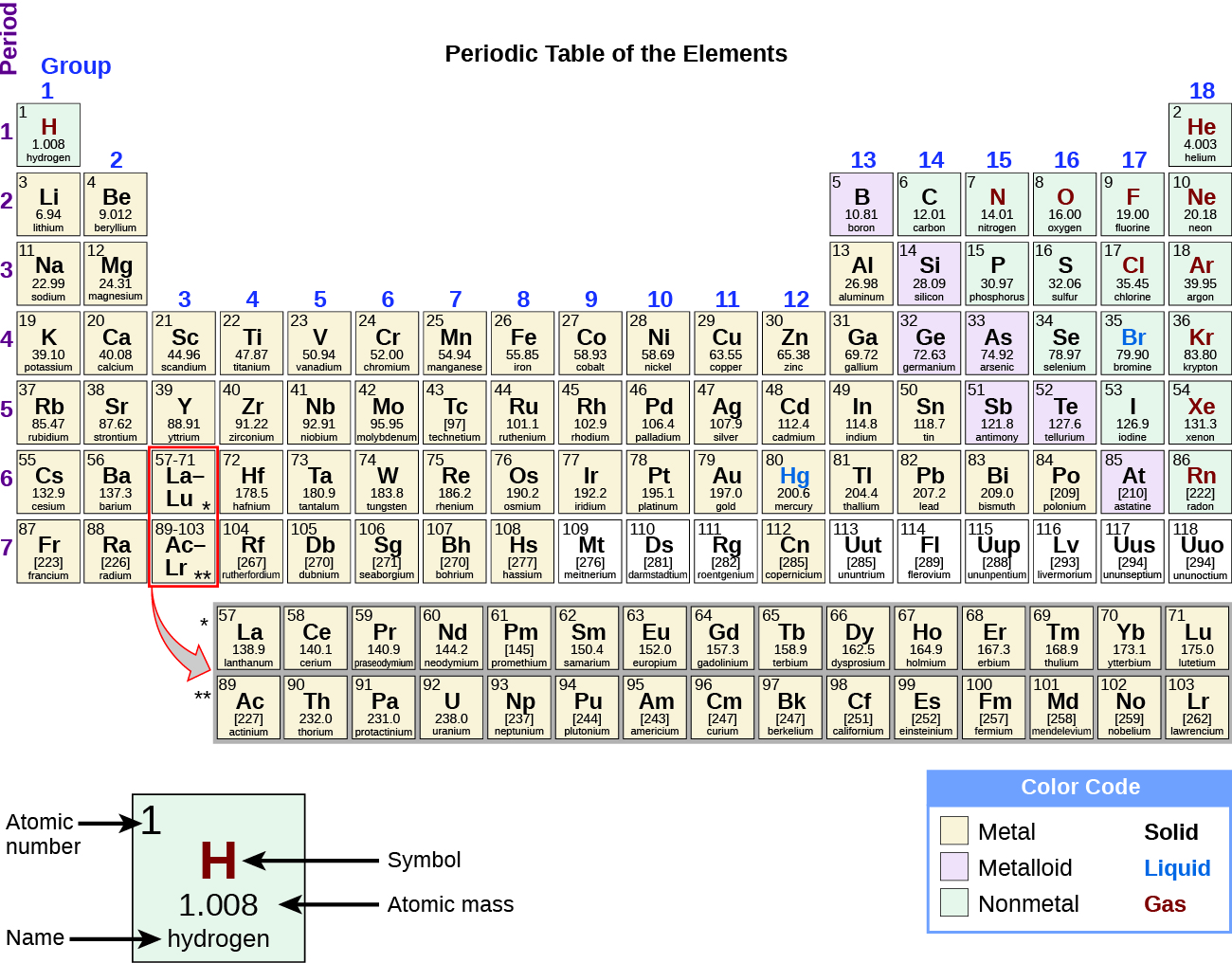
2 5 The Periodic Table Chemistry

Periodic Table Periodicity Of Properties Of The Elements Britannica

0 Comments